Humanizing diabetes management
My role
Design Technologist, Project Lead
Output
Android App
Timeline
October 2017 - October 2018
Industry
Healthcare, medical devices
Program Overview
Partnering with a global pharmaceutical provider with a globally distributed team across three time zones, together we created a class III medical device app for android that allowed individuals with type 1 diabetes to dispense and calculate their insulin over bluetooth.
Named the Pump App, it would successfully pair with any bluetooth ready insulin pump to administer insulin in a way that was both discrete and humane, seeking to improve the relationship between humans and their medical technology.
What were the outcomes?
The Pump App beta release was delivered fully functional with the ability to administer insulin through a bluetooth ready insulin pump. It was designed to be completely accessible, easy to use and was vetted through multiple risk assessments and user testing interviews.
12 months delivering fully functional beta release with 10 person team
3 timezone coordination between client, delivery and safety teams
2 privacy professionals consulted with during design ahead of GDPR
1 beta produced allowing for bolus administration and calculation
What was the value?
Our client needed to be able to prove that it was technologically possible for an insulin pump to connect wirelessly via bluetooth to a mobile phone, simply and safely. We were able to release a beta in under 12 months satisfying these requirements, which allowed our client to learn more about what the market would require to release an app as revolutionary as the Pump app.
1
We created an easy to use app on android for patients to deliver their insulin
How?
To design this app in a way that was tightly coupled with the hardware it was leveraging, I worked hand-in-hand with the technology team using their same lean agile approach to design. This allowed me to quickly iterate on the UI/UX design with new learnings validated by the hardware. We prioritized the proof of functionality first and later added in a finessed UI layer, once we could prove that the app worked successfully with the bluetooth insulin pump in a user friendly way.
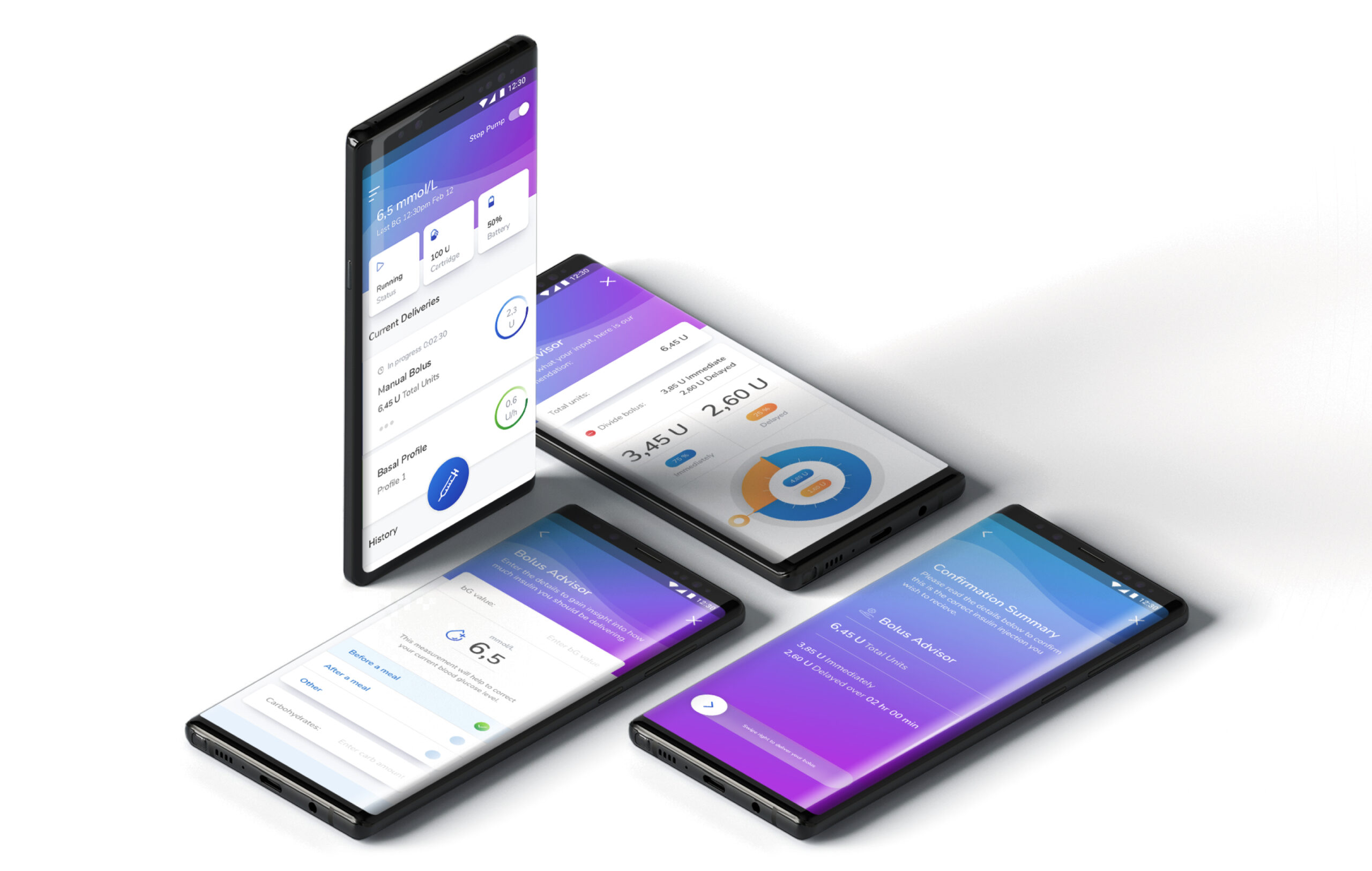
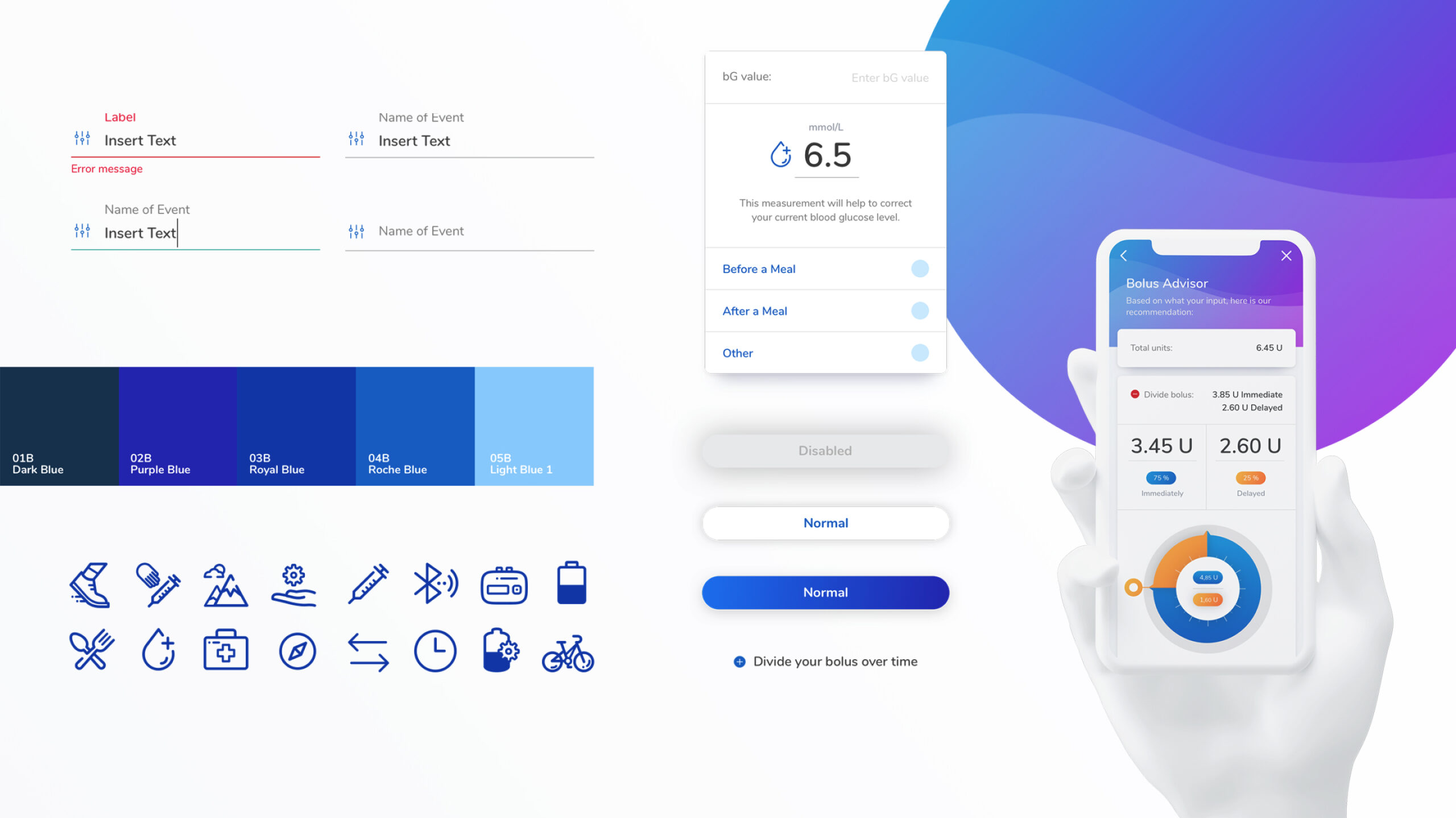
Pictured Above: UI screens from the Pump App including the insulin dashboard, Bolus Advisor, and confirmation screen when administering a bolus.
2
We simplified the experience of using a bolus advisor to calculate insulin injections
How?
To ensure the experience of calculating insulin was as simple as possible for patients, we conducted multiple user testing interviews and feedback sessions. This gave us deep insight into the quality of experience our app was providing. Here are a few video clips from our feedback sessions:
Pictured Above: A parametric tile with a frame from an interview with a good friend and type I diabetic, Dox. Click here to watch the conversation.
3
The app was designed with a GDPR privacy story, ensuring market viability for Europe
How?
Early on we had our GDPR surface as a potential blocker for our release when analyzing the market in Europe. This was possible thanks to our workflow of using an agile scrum methodology, which surfaced issues quickly by including all scrum team members to participate in solutioning. Our product manager had eyes on our market, and was able to research the regulations around releasing a class III medical device in Europe. We quickly brought in Good Research to consult on our user experience, which allowed us to tell a strong privacy story through our user experience.
Pictured Above: UI Screens in the Pump App settings section that points towards data management preferences and privacy settings.
Have questions?
If this is something you would like to know more about, get in touch!
Contact me at
Connect with me at
or read more of my thoughts at
Crafted during a pandemic surrounded by
cat magick, kat noise and cat love.